We will fulfil the promise of a decarbonised future

The Elestor solutions
A rapid transition to a new and entirely clean energy system is now possible, thanks to Elestor’s large-scale flow battery that can store renewable energy for long periods of time.
Learn moreThe fundamentals
Elestor’s flow battery is constructed around an electrochemical cell, where chemical energy is provided by the chemical reaction between two active materials.
Learn moreWorking principle
The active materials are contained in two tanks separated by a membrane stack, through which protons travel to absorb or release electrons during charging or discharging.
Learn moreHydrogen & iron
Both active materials are readily available all over the world, and as they are abundant they are also affordable. They make for a system that is robust, long-lasting and flexible.
Learn more
Scalability
The power [MW] of a flow battery system, as depicted above, is determined by the surface area of the ion-selective membrane, while the capacity [MWh] of the system is determined by the volume of the catholyte and anolyte reservoirs. The fact that the membrane surface area and the reservoir volumes can be dimensioned individually highlights one of the most distinguishing properties of flow batteries, as opposed to traditional electricity storage systems where power [MW] and capacity [MWh] scale simultaneously.
Learn more
Life cycle analysis
We conduct rigorous and ongoing life cycle analysis to evaluate and minimize the environmental footprint of our flow battery technology. Elestor continuously comes up with solutions that help reduce resource consumption, emissions and waste. This improves the sustainability of our products and contributes to a cleaner, greener future.
The flow battery family
Flow batteries can be built around a large variety of chemistries by using different active materials, or so-called redox couples. From a purely scientific perspective, several solutions are workable, yet only some of these can succeed commercially. Before we chose the hydrogen and iron redox couple, we benchmarked it against a complex set of criteria:
Power density
The power density determines the power the flow battery can deliver per square metre of membrane surface area. The lower the power density, the greater the number of square metres of cell materials, especially membranes. High power density thus equals lower costs for the total flow battery system.
Learn more
Energy density
The energy density determines the energy the flow battery can store per cubic metre of active materials. The lower the energy density, the larger the volume of active materials, which means larger tanks, which require more space. High energy density thus equals lower costs for the total flow battery system.
Learn more
Cost of storage
The cost of the active materials, usable capacity, efficiency and lifetime are some of the factors that determine the cost of storage, which in turn determines the economic value of a storage technology, and thus of its market adoption, and finally of its impact on the energy transition.
Learn more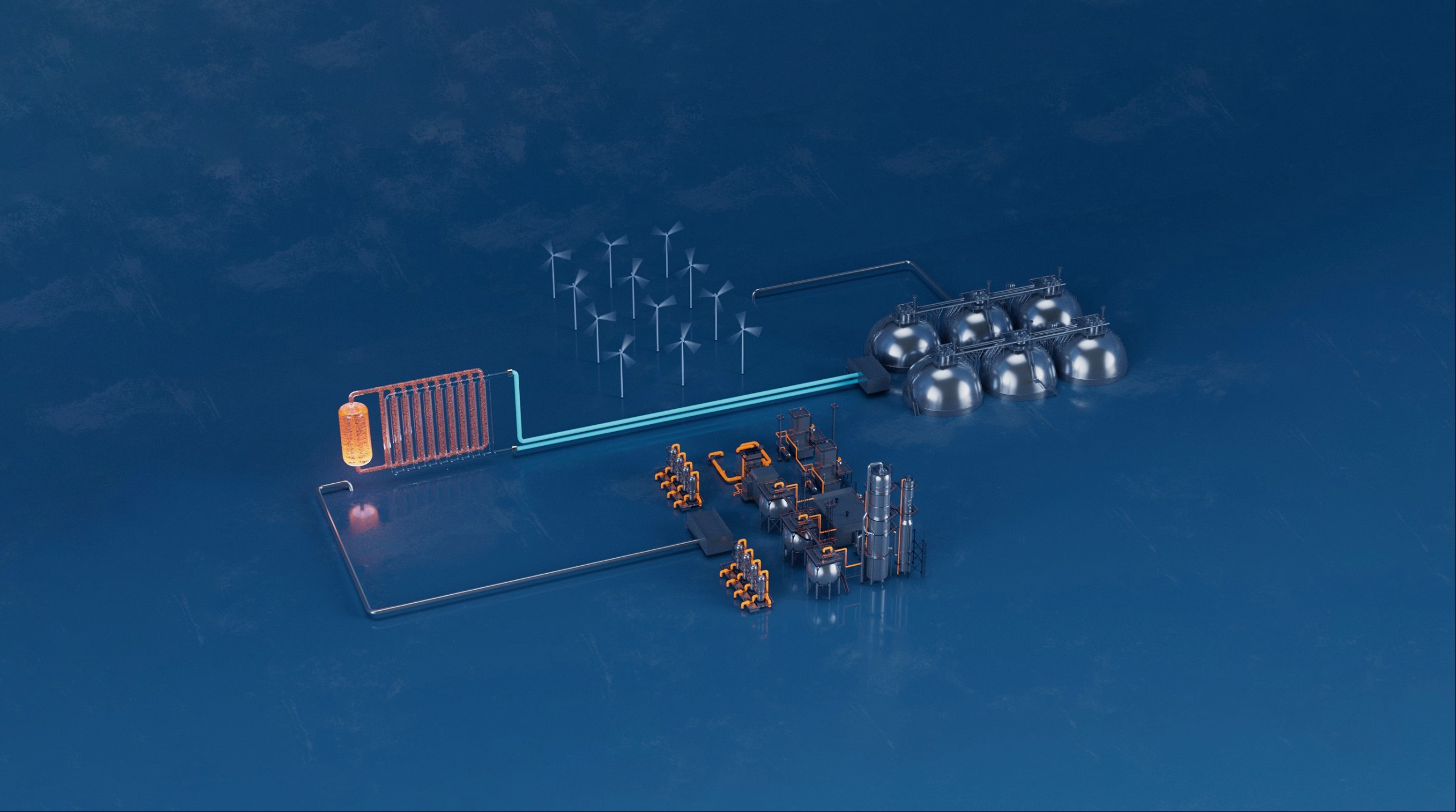
Hydrogen infrastructure
Elestor both benefits from and contributes to the anticipated green hydrogen infrastructure roll-out. We do this by making sure that our flow battery technology can be integrated directly with future hydrogen gas pipe networks in a manner that eliminates the need for separate hydrogen tanks. In turn, this scales back the flow battery system’s physical footprint, curbs capital expenditure and reduces storage costs.
Learn moreLong-term performance of hydrogen-bromine flow batteries using single-layered and multi-layered wire-electrospun SPEEK/PFSA/PVDF membranes
Sanaz Abbasiab, Yohanes Antonius Hugob, Zandrie Bornemanac, Wiebrand Koutb and Kitty Nijmeijer*ac
aMembrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, P.O. Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: D.C.Nijmijer@tue.nl
bElestor BV P.O. Box 882, 6800 AW Arnhem, The Netherlands
cDutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, The Netherlands
Abstract
Sulfonated poly (ether ketone) (SPEEK), perfluorosulfonic acid (PFSA), and polyvinylidene fluoride (PVDF) were wire-electrospun. Subsequently, multiple electrospun layers in different arrangements were hot-pressed into sustainable membranes for use in hydrogen-bromine flow batteries (HBFBs). The relationship between the electrospun layer composition and arrangement, membrane properties, and battery performance was explored. Wire-electrospinning and hot-pressing improved SPEEK and PFSA/PVDF compatibility, yielding dense membranes. Higher SPEEK contents lead to rougher morphologies, while the insulating nature of PVDF decreases the ion exchange capacity (IEC) and HBr uptake compared to commercial PFSA. The multi-layer assembly negatively impacted the membrane transport properties compared to the single-layer arrangement. Although wire-electrospinning improves the polymer dispersion and fixed charge density, SPEEK-rich regions of the blend membranes lack the high selectivity of PFSA, thus reducing the ionic conductivity. This is especially clear in the multi-layer membranes with accumulated SPEEK in the intermediate layer in the through-plane direction. Following initial property comparisons, thinner wire-electrospun SPEEK membranes were prepared with area resistance in the PFSA-comparable range. Among the wire-electrospun SPEEK/PFSA/PVDF membranes, the single-layered membrane with 8 wt% SPEEK (SPF1-8; 62 μm) displayed stable HBFB performance at 200 mA cm−2 over 100 cycles (64 cm2 active area). Based on the ex-situ measurements and cell performance results, a total of ∼10.5 wt% SPEEK is suggested as the limit for both single and multi-layered wire-electrospun membranes, combined with a maximum membrane thickness of ∼50 μm. This ensures robust HBFB performance, positioning wire-electrospun SPEEK/PFSA/PVDF membranes as a PFSA alternative in energy storage.
Wire based electrospun composite short side chain perfluorosulfonic acid/ polyvinylidene fluoride membranes for hydrogen-bromine flow batteries
Yohanes Antonius Hugo a, b, Wiebrand Kout b, Antoni Forner-Cuenca a, Zandrie Borneman a, c, Kitty Nijmeijer a, c, *
a Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, PO Box 513, 5600MB Eindhoven, the Netherlands
b Elestor B.V., 6827 AV Arnhem, the Netherlands
c Dutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, the Netherlands
⁎ Corresponding author. E-mail address: d.c.nijmeijer@tue.nl (K. Nijmeijer).
Abstract
A main component of a hydrogen-bromine flow battery (HBFB) is the ion exchange membrane. Available membranes have a trade-off between the major requirements: high proton conductivity, low bromine species crossover, and high mechanical and chemical stability. To overcome this, electrospinning of a highly proton conductive polymer (short side chain perfluorosulfonic acid (SSC PFSA)) and a hydrophobic inert polymer (polyvinylidene fluoride (PVDF)) was used to electrospin composite polymer fiber mats. Piles of multiple mats were hot pressed resulting in dense ion exchange membranes. Membranes with three different SSC PFSA/PVDF ratios were prepared, characterized, and subjected to short and long term (1500 h) HBFB testing. The electrospun membranes have performances very comparable to those of commercial membranes. For the SSC PFSA/PVDF electrospun membrane, a higher SSC PFSA loading gives a higher membrane proton conductivity compared to a lower loading, but at the expense of a higher bromine species crossover. The SSC PFSA/PVDF (50/50 wt%) membrane shows a coulombic efficiency of 98%, a voltaic efficiency of 80% and an initial available capacity of 105 Ah L− 1 at a current density of 150 mA cm− 2, which equals that of the current benchmark long side chain PFSA membrane. This performance is constant over 200 cycles during 2 months of continuous HBFB operation.
High selectivity-conductivity reinforced perfluorosulfonic acid membranes for hydrogen-bromine flow batteries.
Yohanes Hugo1, 2, Wiebrand Kout1, Friso Sikkema1, Zandrie Borneman2, Kitty Nijmeijer2
1Elestor B.V., 6812 AR Arnhem, the Netherlands
2Membrane Materials and Processes, Eindhoven University of Technology, Department of Chemical Engineering and Chemistry, PO box 513, 5600 MB Eindhoven, The Netherlands
Abstract
Reinforced proton exchange membrane (PEM) were developed to increase the mechanical strength of thin membranes (≤30 µm) for PEM fuel cell applications. In hydrogen-bromine flow batteries (HBFBs), Br2 and Br– crossover through the membrane may affect the lifetime of HBFBs as a result of dissolution or passivation of the platinum catalyst. One study suggested that the reinforcement reduces the bulk Br2 and Br– transport and x-y (in-plane) swelling [1].
Effect of Bromine Complexing Agents on Membrane Performance in Hydrogen Bromine Flow Batteries
Yohanes Antonius Hugo1,2, Natalia Mazur2, Wiebrand Kout2, Guido Dalessi2, Antoni Forner-Cuenca1, Zandrie Borneman1, 3 and Kitty Nijmeijer1,3,*
1Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, P.O. Box 513, 5600 MB Eindhoven, The Netherlands; yohanes.hugo@elestor.nl (Y.A.H.); a.forner.cuenca@tue.nl (A.F.-C.); z.borneman@tue.nl (Z.B.)
2 Elestor B.V., P.O. Box 882, 6800 AW Arnhem, The Netherlands; wiebrand.kout@elestor.nl (W.K.); guido.dalessi@elestor.nl (G.D.)
3 Dutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, The Netherlands
Abstract
The addition of bromine complexing agent (BCA) to bromine electrolyte is an accepted method to reduce bromine vapor pressure making bromine-based flow batteries inherently safer. It is well-known that the amine functional group of the BCAs interact with Nafionmembranes.The novelty of the current work is that it investigates how this interaction of BCA with the four different membrane chemistries impacts the membrane characteristics and performance of hydrogen bromine flowbatteries(HBFBs). The impact of BCA 13 on the system performance is determined by the membrane chemistry. Exposure of Nafion membranes to BCA leads to 60% higher cell resistance, and 55% lower cell power density at 0.5V at 50% state-of-charge(SOC). This decrease is caused by the strong interaction between the negatively charged sulfonic acid groups in the membrane and the positively charged BCA. Lower SOC, lower bromine concentration and a higher free BCA concentration is detrimental in the cell operation. The use of LC PFSA membranes in the presence of BCA ions should be avoided. while BCA in combination with grafted sulfonated polyvinylidene fluoride (SPVDF) or grafted sulfonated polyethylene (SPE) membranes promising HBFB results are obtained.
The effect of cations on the proton transport of PFSA membranes used in hydrogen-bromine flow batteries: observations and mitigation solutions.
Natalia Mazur1*, Yohanes Antonius Hugo1,, Wiebrand Kout1, Friso Sikkema1, Ran Elazari2, Ronny Costi2
1 Elestor B.V., Arnhem 6812 AR, The Netherlands
2 ICL Industrial Products R&D, Beer Sheva, Israel
*natalia.mazur@elestor.nl
Abstract
In search for cheap, high capacity energy storage, hydrogen-bromine flow batteries (HBFBs) are emerging as strong contenders [1], however, the volatility of the electrolyte and the associated risks must be managed. Bromine complexing agents (BCAs) are used as additives to the electrolyte, which have the ability to capture Br-ions and Br2 thus decreasing the bromine vapour pressure [1]. The BCA-HBr-Br2 complex separates as a high density oily layer.
Performance mapping of cation exchange membranes for hydrogen-bromine flow batteries for energy storage
Yohanes Antonius Hugo a, b, Wiebrand Kout b, Antoni Forner-Cuenca a, Zandrie Borneman a, c, Kitty Nijmeijer a, c, *
a Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, PO Box 513, 5600MB Eindhoven, the Netherlands
b Elestor B.V., 6827 AV Arnhem, the Netherlands
c Dutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, the Netherlands
⁎ Corresponding author. E-mail address: d.c.nijmeijer@tue.nl (K. Nijmeijer).
Abstract
Electricity storage is essential for the transition to sustainable energy sources. Hydrogen-bromine flow batteries (HBFBs) are promising cost-effective energy storage systems. In HBFBs, proton exchange membranes are required to separate the two reactive materials, enabling proton transport for charge balancing. In this paper, we present a comprehensive overview of the key properties and an experimental performance map of cation exchange membranes for HBFBs. Our study shows that membrane water uptake is an important property due to its strong correlation with membrane resistance and bromide species crossover. Long chain perfluorosulfonic acid (LC PFSA) membranes are shown to have a better power density–crossover tradeoff and a higher stability than other types of functionalized membranes. The good power density-crossover tradeoff of LC PFSA membranes is the result of the high level of separation of hydrophobic and hydrophilic domains in the membrane, leading to well-connected ionic pathways for proton transport. Reinforcement of long chain LC PFSA membranes further improves their tradeoff because it mechanically constrains the swelling (lower water uptake), resulting in a lower crossover but a similar peak power density. Consequently, reinforced LC PFSA membranes are the most promising option for HBFBs.
State-of-the-art membranes for hydrogen-bromine flow batteries: The mechanisms for proton and bromide-species transports
Yohanes Antonius Hugo1, Wiebrand Kout1, Kitty Nijmeijer2
1Elestor B.V., Arnhem 6812 AR, the Netherlands
2Membrane Science & Technology Group, Department of Chemical Engineering,
Eindhoven University of Technology, Eindhoven 5600 MB, the Netherlands
Abstract
In a hydrogen-bromine flow battery (FB), the membrane characteristics determine the intrinsic resistance and crossover rate of bromide species and water. The crossover issue (from the bromine side to the hydrogen side) affects the performance and lifetime of hydrogen-bromine FBs because the bromide species deactivate the platinum catalyst and the liquid floods the electrode, which makes it inaccessible for the hydrogen [1].
Low-cost wire-electrospun sulfonated poly(ether ether ketone)/poly (vinylidene fluoride) blend membranes for hydrogen-bromine flow batteries
Sanaz Abbasia, b, Antoni Forner-Cuencaa Wiebrand Koutb, Kitty Nijmeijer a, c, Zandrie Borneman a, c, *
a Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, PO Box 513, 5600MB Eindhoven, the Netherlands
b Elestor B.V., 6827 AV Arnhem, the Netherlands
c Dutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, the Netherlands
⁎ Corresponding author. Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, P.O. Box 513, 5600 MB, Eindhoven, the Netherlands. E-mail address: Z.Borneman@tue.nl (Z. Borneman).
Abstract
Cost-effective dense membranes were developed by blending proton-conductive sulfonated poly(ether ether ketone) (SPEEK) with inert, mechanically stable poly(vinylidene fluoride) (PVDF) for hydrogen-bromine flow batteries (HBFBs). Wire-electrospinning followed by hot-pressing was employed to prepare dense membranes. Their properties and performance were compared to solution-cast membranes of similar composition and thickness. Electrospinning improved the ionic conductivity and bromine diffusion properties by providing interconnected ion-conductive SPEEK nanofiber pathways through a PVDF matrix. Relatively thin (~50–60 μm) electrospun membranes with a SPEEK/PVDF ratio (wt%/wt%) of 90/10 and 80/20 showed comparable Br3 − diffusion rates as the relatively thick and commercially available perfluorosulfonic acid (PFSA) membrane (~100 μm) at a 35%–42% lower proton conductivity. The latter can be attributed to the poorer ion conductivity of SPEEK compared to PFSA and the presence of PVDF. The HBFB single cell featured the best polarization behavior and ohmic area resistance with the electrospun membrane containing 80/20 (wt%/wt%) SPEEK/PVDF. However, the low thickness and insufficient chemical/mechanical stability of the ES 80/20 causes a rapid decay in the HBFB cycling performance. This study promotes a life-time comparison study between the low-cost wire- electrospun SPEEK/PVDF blend membranes (~€100 m− 2) and the typically used PFSA membranes (~€400 m− 2) for a long-term HBFB performance.
In situ long-term membrane performance evaluation of hydrogen-bromine flow batteries
Yohanes Antonius Hugo a, b, Wiebrand Kout b, Kitty Nijmeijer a, c, *, Zandrie Borneman a, c
a Membrane Materials and Processes, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, PO Box 513, 5600MB Eindhoven, the Netherlands
b Elestor B.V., 6827 AV Arnhem, the Netherlands
c Dutch Institute for Fundamental Energy Research (DIFFER), P.O. Box 6336, 5600 HH Eindhoven, the Netherlands
⁎ Corresponding author. E-mail address: d.c.nijmeijer@tue.nl (K. Nijmeijer).
Abstract
Because of the impracticality of long life time testing of hydrogen bromine flow batteries (HBFBs) under real, cyclic operating conditions, we utilized a high-frequency cycling load accelerated lifetime test (ALT) to evaluate the membrane electrode assembly performance in HBFBs. Four different membrane chemistries were tested to assesstherelativelong-termperformanceanddurabilityofthecorrespondingHBFBsandtounderstand themain long-term failure mechanism in HBFB technology. The high-frequency cycling load ALT is a highly valuable method to assess long-term HBFB (components) stability and to understand the degradation/failure mechanism. The results showed that the utilization of long side chain perfluorosulfonic acid (LSC PFSA, i.e. Nafion®) membranes result in stable HBFB operation until a sudden cell failure at the end of the cycle life. The use of a more selective (lower bromine species crossover) reinforced LSC PFSA membrane results in approx. five times longer life times. In contrast, the use of a grafted polyvinylidene fluoride (SPVDF) membrane results in a slow incremental performance decrease (or area resistance increase) during the ALT and, again, a sudden cell failure at the end of the cycle life. After the ALT, the LSC PFSA membrane still shows high chemical stability. On the other hand, the grafted SPVDF and SPE membranes show clear membrane degradation visible as a strong decrease in IEC and an increase in ohmic AR. Gradual Pt catalyst degradation-dissolution, that results in insufficient Pt catalyst loading and subsequently low hydrogen reaction kinetics, is the main failure mechanism of the HBFBs. The membrane bromine species crossover rate is directly related to the rate of Pt catalyst degradation-dissolution and scales almost linearly with the celltotal ampere-hours. Aslong as thecatalyst loading issufficientanddoes not reacha minimumvalue,this observed Pt degradation-dissolution rate does not significantly impact the cell performance.

